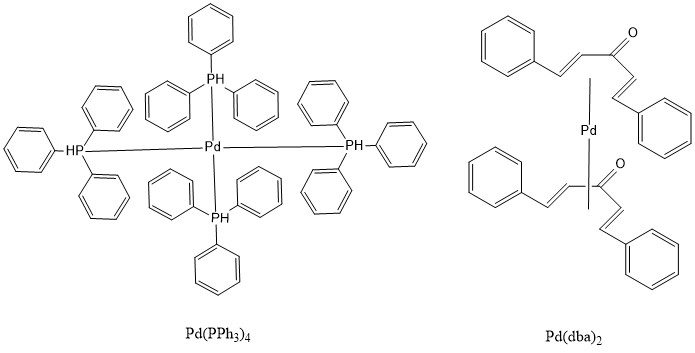
Catalyst tailoring for palladium-mediated cross coupling of arylstannanes with vinyl triflates - ScienceDirect

Palladium-Catalyzed Regio- and Diastereoselective Allylic Alkylation with Azlactones Using Triphenylarsine

Preparation and catalytic properties of the triphenylarsine and triphenylstibine-stabilized tri-heteroleptic NHCPd‒allyl complexes - ScienceDirect
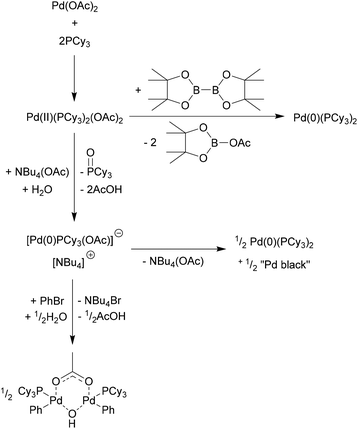
Solvent effects in palladium catalysed cross-coupling reactions - Green Chemistry (RSC Publishing) DOI:10.1039/C9GC00617F
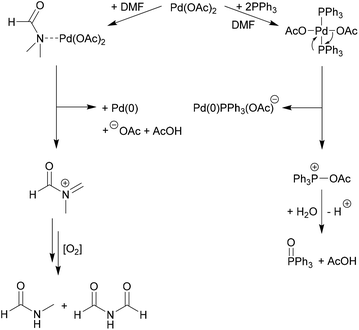
Solvent effects in palladium catalysed cross-coupling reactions - Green Chemistry (RSC Publishing) DOI:10.1039/C9GC00617F

PDF) Neutral Palladium(0) complexes from Pd(OAc)2 and Tri-2-furylphosphine and their reactivity in oxidative addition with PhI

PDF) Selective palladium-mediated synthesis of racemic 4,5-disubstituted 5H-furan-2-ones from 3-ynoic acids and organic halides | Fabio Bellina - Academia.edu

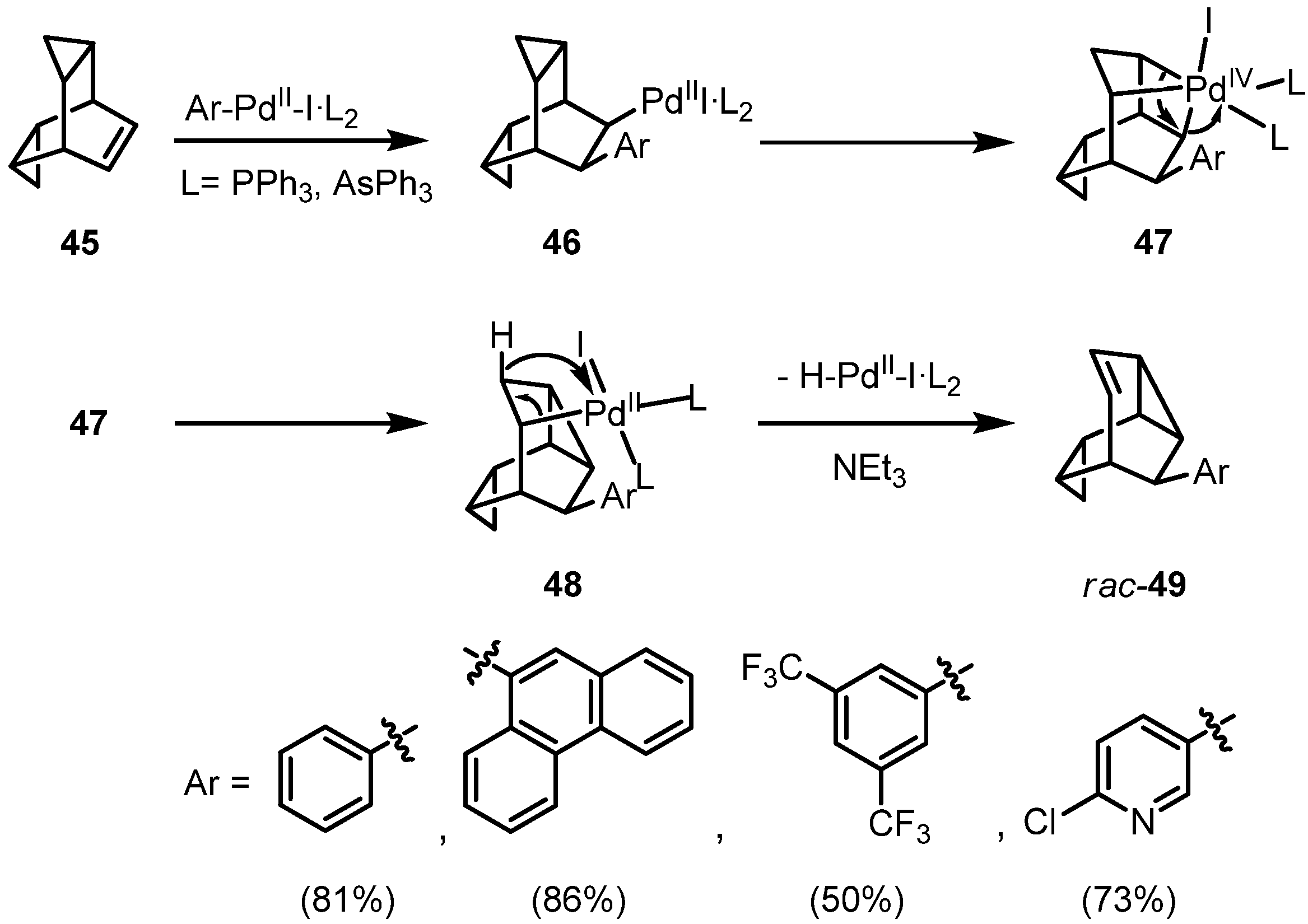
Intramolecular C−H Activation by Alkylpalladium(II) Complexes: Insights into the Mechanism of the




/13EF7E15675831C4802585FA0044F15F/$file/FT61307_structure.png)