Zacks Industry Outlook Highlights: Dr. Reddy's Laboratories, Teva Pharmaceutical and Amphastar Pharmaceuticals

Rudolph's Shoe Mart Teva Pharmaceutical Industries Sandal Golden Shoes, PNG, 500x500px, Teva, Area, Black And White,

Teva Branded Pharmaceutical Products R&D;, . An electronic inhaler add-on module which store and transmit information related to inhaler use (eModule) PDR0000401 FCC ID 2AJVSPDR0000401
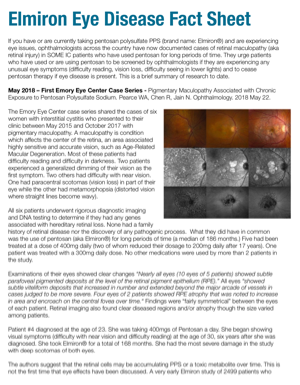
First Lawsuit Filed Over Elmiron Eye Damage - Pelczar v. Teva Branded Pharmaceuticals R&D et al - Interstitial Cystitis Network